03 | Funded Project
TAMEP and their progenitor cells control glioblastoma hallmarks

Funding Period: 01.01.2026 – 31.12.2027
Project Lead: Prof. Dr. Rainer Glaß
University Hospital for Neurosurgery Munich
Project Description
Throughout the last years funding by the Anni Hofmann Stiftung enabled us to gain unique insights into the brain tumor supporting function of both TAMEP-progenitors and their more differentiated progeny (named TAMEP). Overall, this cell lineage has remarkable impact on GBM progression in a two-step process: First, TAMEP-progenitors are required to prime the extracellular matrix (matrisome) for pathological angiogenesis and BTB formation. Second, TAMEP attract myeloid cells into GBM aggravating the inflammatory state of GBM. The TAMEP-progenitors promote GBM vascularization and vessel tightness by activating enzymes with matrisome crosslinking activity. Based on this discovery we are now starting to therapeutically leverage fully translational procedures tackling angiogenesis and BTB formation. As one objective for this funding period we envision that combined blockade of matrisome crosslinking enzymes will provide a new, preclinical paradigm for improved GBM chemotherapy.
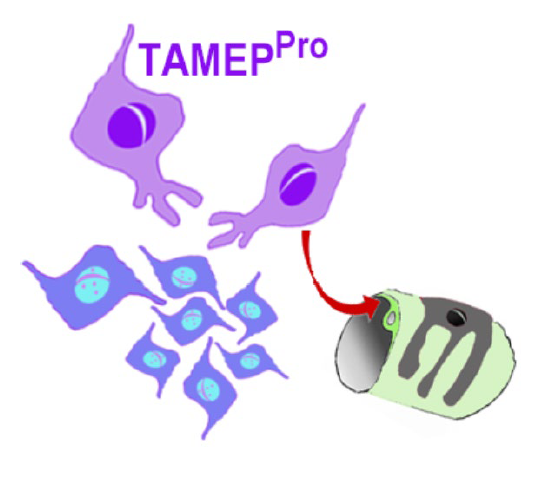
Interaction of TAMEP Progenitor-cells (TAMEP PRO) with GBM cells (depicted in blue) and the tumor vasculature. In early stages of gliomagenesis parenchymal progenitors can foster malignant progression of the tumor primordium. In later stages of GBM growth and in relapsing tumors TAMEPPRO control tumor vascularization and formation of the blood-tumor-barrier (BTB); emblemized by the arrow. The BTB obstructs entry of chemotherapeutics into GBM.
Furthermore, we will explore TAMEP-induced brain tumor inflammation and fibrosis, which are central mediators for GBM cell invasion as well as therapy resistance. Currently, it is not yet clear how TAMEP control these important pathological pathways. We have observed that TAMEP actively attract myeloid cells into GBM, the increased density of such tumor associated myeloid cells may then result in augmented fibrotic inflammation. However, detailed knowledge on the signaling hierarchies controlling this inflammatory cycle is a prerequisite for therapeutic intervention (which ideally targets key points of the signaling cue). Hence, we will investigate the TAMEP-related signal transduction and cell-cell interaction networks in order to design new treatment paradigms interfering with inflammatory fibrosis in GBM.
